Introduction
Induction chemotherapy (IC) in acute myeloid leukemia (AML) in younger and fit patients has historically combined an anthracycline (ie, daunorubicin or idarubicin) with the antimetabolite cytarabine, which has been termed the 7 + 3 regimen. Response rates with this current standard approach show complete response rates of 70-80%, however 30-40% still eventually relapse. Recently, the MD-Anderson group published a phase II study in which the drug venetoclax was added to the standard anthracycline/cytarabine induction regimen of FLAG-IDA. Results from this study showed an impressive 96% response rate for newly diagnosed (ND) AML patients. Based on these results we began to adopt this approach for our patients. We have induced 21 patients with this approach at our institution and have had comparable results.
No study has compared outcomes between this approach and that of standard 7 +3 in regards to response rates. Based on our improved outcomes we compared outcomes between these two approaches in our patient population by doing a retrospective chart based review on all patients that received cytotoxic induction treatment for ND-AML between 2000-2023.
Methods
Patients between 2020-2023 that were treated with induction cytotoxic chemotherapy for AML, based on WHO 2016 criteria, were retrospectively reviewed. Patients either received 7+3 or 7+3 plus midostaurin if they were FLT3+ in one arm or FLAG-IDA/Venetoclax. Patients received FLAG-IDA/Venetoclax regardless of FLT3+ status or risk of disease. Patients with extra medullary disease were excluded from this review as well as patients that received an IDH1 or 2 inhibitors in combination with 7 +3.
Results
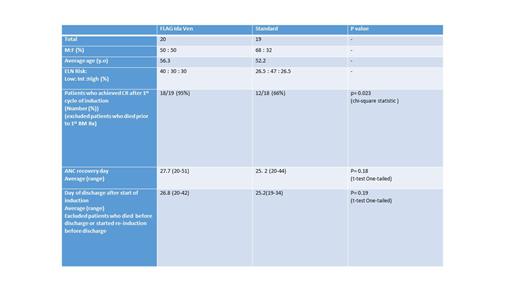
Overall 39 patients were included in this study, 20 patients in the FLAG-IDA/Venetoclax arm and 19 patients in the 7/3 arm. In patients which response was able to be evaluated patients in the FLAG-IDA/Venetoclax cohort had a 100% overall response rate with a 95% (N = 19/20) complete response rate. Patients in the 7/3 cohort the complete remission rates were 66% (N = 12/16) with 3 of the patients achieving a complete remission with re-induction. Sixty-day morality rate was 5% (N = 1/20) in patients receiving FLAG-IDA/Venetoclax compared to 16% (N = 3/19). In terms of neutrophil recovery and toxicity results were comparable in both arms, with the average day to cell recovery being the similar in both groups.
Conclusions
FLAG-IDA/Venetoclax compared to standard 7/3 +/- FLT3 directed therapy in our patient population resulted in improved response rates and decreased 60-day mortality. Our results are similar to previously published reports in patents with ND-AML. Toxicity profiles were similar with the clear benefit of decreased early morality are increased response rates.
Disclosures
Varshavsky-Yanovsky:Janssen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal